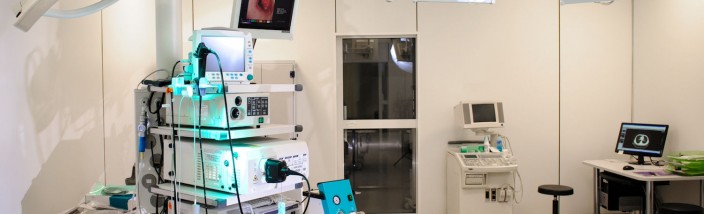
Electromagnetic Navigation Bronchoscopy – pro
ENB (Electromagnetic Navigation Bronchoscopy) or EMN bronchoscopy is a type of bronchoscopy that uses electromagnetic guidance to project catheters into and through bronchial passages. Using a virtual, three-dimensional (3D) bronchial map from a recent CT scan and disposable catheters, it makes it possible to navigate to a desired location within the lung and to look at it or biopsy it, stage lymphatic nodes or to insert markers to guide future
Read more